Dealing with contamination doubts before taking on new customers
Mike O’Brew (name changed for the confidentiality of the business) is a successful microbrewery where all employees know how essential hygiene is to beer quality and spend a large proportion of their time cleaning and looking out for anything suspicious.
Batches discarded because of contamination are rare, estimated at less than 1%, but customer feed-back suggests that some contamination issues might occur once in a while, during storage. On occasion, the brewery now produces for other brands and has decided to improve its practices to meet the demand of this new type of customers.
The vast majority of the equipment is cleaned with caustic prior to sanitization with peracetic acid, rinsed, then closed after disinfecting the plugs with a spray of alcohol. The majority of the Clean-in-Place (CIP) protocol is done using a mobile CIP unit with removable connectors kept in PA when not in use. A few pieces of equipment such as the hop infusion system are cleaned with caustic, allowed to dry, then sanitized and rinsed immediately prior to use.
When the new guy has ideas (about hygiene monitoring)
Mike O’Brew had recently hired Glenn as Quality Assurance manager with experience in brewing and as one of the rare guys with short hair among the staff.
After a few months of reviewing practices, CIP cycles and solutions, suppliers, the equipment, and having changed what most needed to, the next step for Glenn was to monitor the process, to collect evidence to help set the next priorities and facilitate the adoption of the new practices by the personnel.
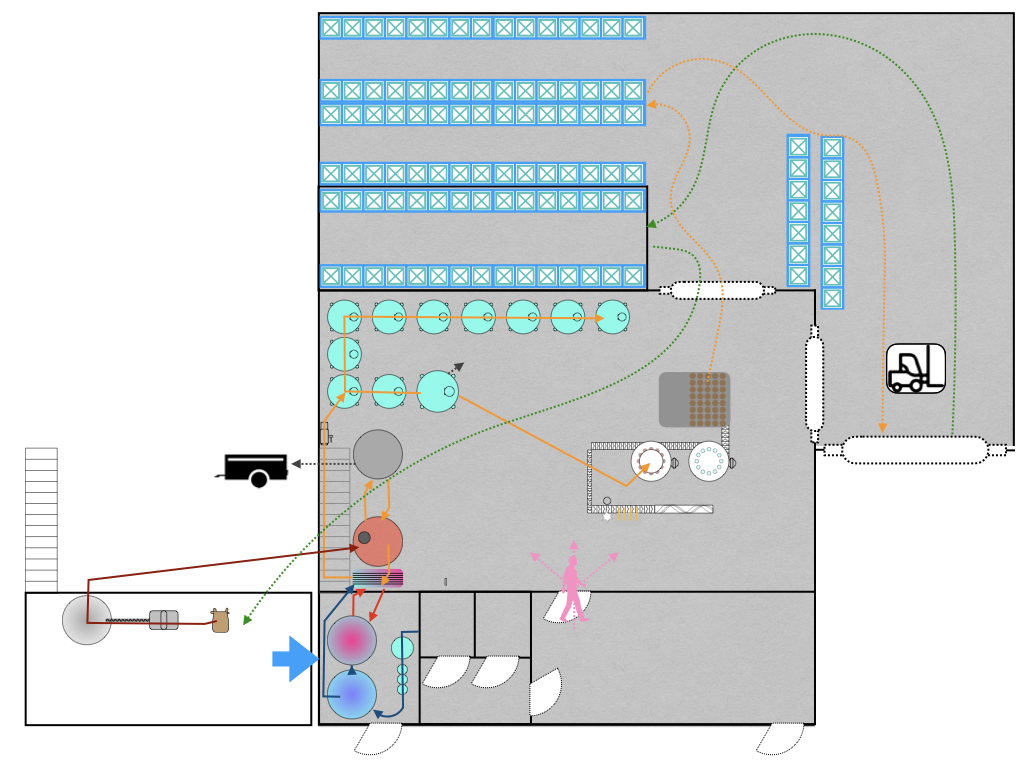
Testing sensitive points in the brewing process to look for clues
Glenn decided to:
- monitor key brewing steps and in particular CIP efficiency, to establish a baseline for residual contamination after sanitization, set alert levels, then improve the practices or design for the steps that are least satisfying.
- start surveillance of the process environment. He was conscious that most of the steps, from milling to bottling are conducted in the same area, which increases the risks of cross-contamination, and that the building configuration leaves the equipment vulnerable to contamination from air, raw-material inputs and waste management.
- lift existing doubts, specifically:
- the sanitary status of the bottling line filling heads after CIP
- the transfer of the wort from the kettle to the fermentor via the heat exchanger
- confirm the rapid ATP tests were sufficiently sensitive to detect contamination in the tank CIP rinse water at a concentration where they might regrow between tank sanitization and fermentation
- the level of (re-)contamination of the hop infusion system during storage
Collecting data for better microbial monitoring in the future
The project aims to collect preliminary data, then use them to design a relevant monitoring program to monitor the process and locate the biggest risks of cross-contamination.
Working with monitoring experts while keeping autonomy
Glen asked for BioMire’s Quick-Start Microbiology Service for 2 main reasons:
- a third party was expected to have an independent opinion and a methodology for risk assessments
- to save time in setting up the monitoring plan
Prepping for a systematic review
The first step consisted in a systematic review of the Mike O’Brew’s:
- inputs
- process layout
- people, material and process flows
- beer types
- preventive measures in place to control the risk of contamination, such as pest control, water treatment, hygiene practices
- history of borderline and out-of-specification (OOS) batches,
During the 90 minutes video call, Glenn also shared some of his concerns and the doubts that he would like to lift. Relevant sampling points were discussed for the preliminary assessment.

High microbial counts raise suspicion
The following discovery points were sampled and tested, and the results shared with us through Mike O’Brew’s Online account:
- The hop infusion system
Where: approximatively 2 inches inside the system outlet, swabbed on twice the circumference
Counts: 20 bacteria & 5 mold, approximatively 30 cfu per cm2 - The fermentor CIP rinse water
Where: the last volume drained from the tank was collected and tested
Counts: 8 cfu /ml - The bottling line
Where: bottle neck seal from 3 filling head were swabbed once around
Counts: 92 bacteria & 2 molds, approximatively 30 cfu / cm
The transfer from boiler to fermentor
Where: the flexible pipe was disconnected from the fermentor and the first volume of wort collected in the test device
Count: 0, so < 1 cfu/ml
Narrowing down to specific equipment at high risk
The On-The-Fly results suggested that 2 pieces of equipment should be carefully monitored in the upcoming monitoring plan design:
- The bottling line
- The fermentors
Given the importance and criticality of the wort cooling and transfer, the sampling would be continued until the data history would prove it unnecessary.
The result from the hop infusion system was « good to know », but did not require immediate action, since peracetic acid is efficient on bacteria, yeast and mold at the T° and contact time applied during the sanitization.
The subsequent monitoring plan was influenced by these findings with a particular emphasis on the bottling line (carters, conveyors, capper, bottle feed, ..) and fermentors (man-hole, connectors, rinse water quality, etc.) for 3 months to complete the risk assessment and establish the baseline for normal operating conditions.
Over the following months, Glenn’s doubts were going to be lifted, one way or the other!
Humidity and beer residue help microorganisms grow
Probable cause for the filling head bottle neck seals to be contaminated only minutes after the hot CIP rinse water was run through the equipment?
- The CIP solutions run through the bottler and pours out of the filling head via gravity.
The contact with the entire seal surface is not complete, potentially leaving beer residue which constitute nutrients for most environmental microbes from the brewery - Likewise, the seals are not dried by the compressed air pushed through the equipment before it goes idle. In the presence of water and fed by nutrients, microorganisms have had the opportunity to develop a biofilm which is then resistant to even advanced CIP cycles.
Insights:
- This case is a reminder that water supports life: give them even a small chance and microorganisms will grow when there is water, hence drying equipment and ensuring full contact of sanitants with all humid surfaces are important to ensure perfect equipment hygiene.
Anticipating additional problems
The insights from Mick O’Brew’s case may alert us to other equipment for which complete sanitization or drying may be difficult and open the door to contaminations:
- Water treatment systems
- storage tanks without a sprinkler ball for sanitation
- plant floors or equipment parts difficult to access with a squeegee or a dry cloth
Microbe-wise – A quick look at Lactobacillus
Lactobacillus belongs to the group of lactic acid bacteria, which also includes pediococcus. It is a Gram-positive, aerotolerant anaerobes or microaerophilic, non-spore-forming bacteria capable of forming biofilms allowing them to persist during harsh environmental conditions and maintain ample populations.
They grow best in environments with a pH value of 4.0 to 5.0 and a temperature of roughly 30°C (85°F). Some species of lactobacillus have a high tolerance for the presence of hop compounds and can survive under anaerobic conditions.
Lactobacillus metabolize sugars and produce lactic acid which may cause notable off-flavors in most types of beer.
When grown at ambient temperature, lactobacillus form small colonies appearing after 7 days incubation
Disclaimers
This Use Case is based on a true story! (Though we edited it for readability and, of course, for confidentiality.) We like to share stories about real situations because we think they help translate raw facts into helpful insight and understanding. We’re providing this use case as an illustration, but it does not imply that the same conclusions can be made in similar cases. We will be happy to discuss it, however, as well as how the particulars of this case might be transferable to your situation—so don’t hesitate to contact us! Also, please be aware that we do sometimes use automatic translations, which might slightly distort the information.


